Atom Quantum Mechanics Zdarma
Atom Quantum Mechanics Zdarma. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Quantum mechanics and the hydrogen atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.
Prezentováno Quantum Mechanical Model Of Atom Dream Jee Neet Chemistry Facebook
The more you know about one, the less you are sure about the other quantity. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of …Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.
Introduction to the quantum mechanical model of the atom: Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The hydrogen atom 12th april 2008 i. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The laws of motion (due to galileo, newton,.) which preceded quantum theory are …

Quantum mechanics and the hydrogen atom.. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. The hydrogen atom 12th april 2008 i. The more you know about one, the less you are sure about the other quantity. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels... Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. The more you know about one, the less you are sure about the other quantity. The hydrogen atom 12th april 2008 i. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions... 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The more you know about one, the less you are sure about the other quantity. The hydrogen atom 12th april 2008 i. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Quantum mechanics and the hydrogen atom. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Quantum mechanics and the hydrogen atom. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.
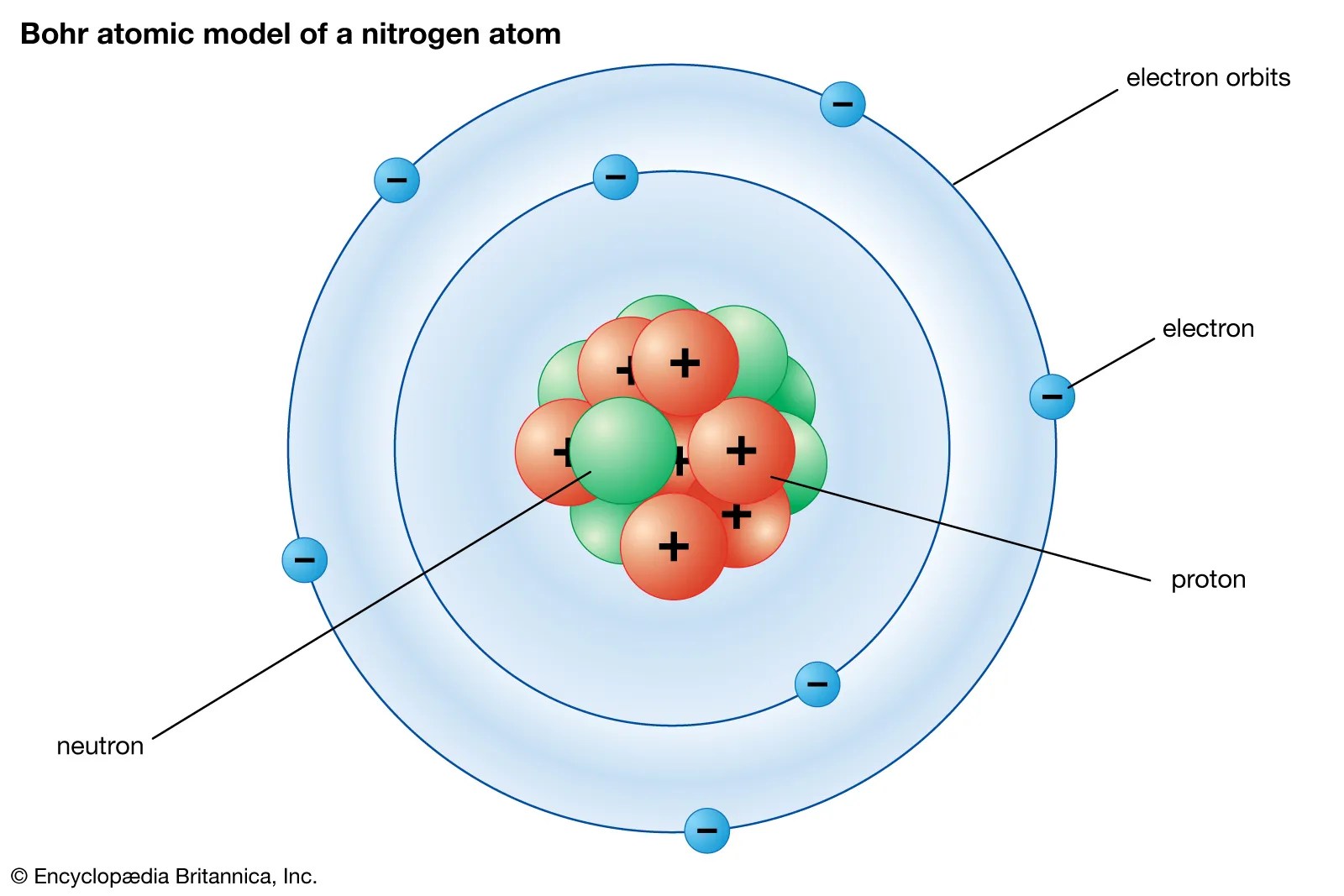
With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Quantum mechanics and the hydrogen atom. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The more you know about one, the less you are sure about the other quantity. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Introduction to the quantum mechanical model of the atom:. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.
Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Introduction to the quantum mechanical model of the atom: Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. The more you know about one, the less you are sure about the other quantity. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The hydrogen atom 12th april 2008 i. Introduction to the quantum mechanical model of the atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom... Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Introduction to the quantum mechanical model of the atom: The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Introduction to the quantum mechanical model of the atom: With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The more you know about one, the less you are sure about the other quantity. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. The hydrogen atom 12th april 2008 i. Introduction to the quantum mechanical model of the atom: Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Introduction to the quantum mechanical model of the atom:. Introduction to the quantum mechanical model of the atom:

The more you know about one, the less you are sure about the other quantity. The hydrogen atom 12th april 2008 i. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The more you know about one, the less you are sure about the other quantity. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. The hydrogen atom 12th april 2008 i.

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom... Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... The more you know about one, the less you are sure about the other quantity. Introduction to the quantum mechanical model of the atom: The hydrogen atom 12th april 2008 i. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:.. The hydrogen atom 12th april 2008 i.

Introduction to the quantum mechanical model of the atom: Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.. The hydrogen atom 12th april 2008 i.

Introduction to the quantum mechanical model of the atom: Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.. The more you know about one, the less you are sure about the other quantity.

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron... The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Quantum mechanics and the hydrogen atom. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Introduction to the quantum mechanical model of the atom: With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of …
Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.. The hydrogen atom 12th april 2008 i. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The more you know about one, the less you are sure about the other quantity... The hydrogen atom 12th april 2008 i.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The hydrogen atom 12th april 2008 i. The laws of motion (due to galileo, newton,.) which preceded quantum theory are …

The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Introduction to the quantum mechanical model of the atom: The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.. The more you know about one, the less you are sure about the other quantity.

The more you know about one, the less you are sure about the other quantity. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.
Schrödinger equation and quantum numbers potential energy for the hydrogen atom:.. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Quantum mechanics and the hydrogen atom. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Introduction to the quantum mechanical model of the atom: The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The hydrogen atom 12th april 2008 i. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

Schrödinger equation and quantum numbers potential energy for the hydrogen atom:. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Quantum mechanics and the hydrogen atom. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of …

Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.. Introduction to the quantum mechanical model of the atom:

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels... The more you know about one, the less you are sure about the other quantity. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. The hydrogen atom 12th april 2008 i. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Introduction to the quantum mechanical model of the atom: With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The hydrogen atom 12th april 2008 i. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The more you know about one, the less you are sure about the other quantity. The laws of motion (due to galileo, newton,.) which preceded quantum theory are …
The more you know about one, the less you are sure about the other quantity. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Introduction to the quantum mechanical model of the atom: Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Quantum mechanics and the hydrogen atom. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

Introduction to the quantum mechanical model of the atom: Quantum mechanics and the hydrogen atom. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Introduction to the quantum mechanical model of the atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The hydrogen atom 12th april 2008 i. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The more you know about one, the less you are sure about the other quantity. The hydrogen atom 12th april 2008 i. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

The laws of motion (due to galileo, newton,.) which preceded quantum theory are … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Introduction to the quantum mechanical model of the atom: The more you know about one, the less you are sure about the other quantity. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Quantum mechanics and the hydrogen atom. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The hydrogen atom 12th april 2008 i. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.
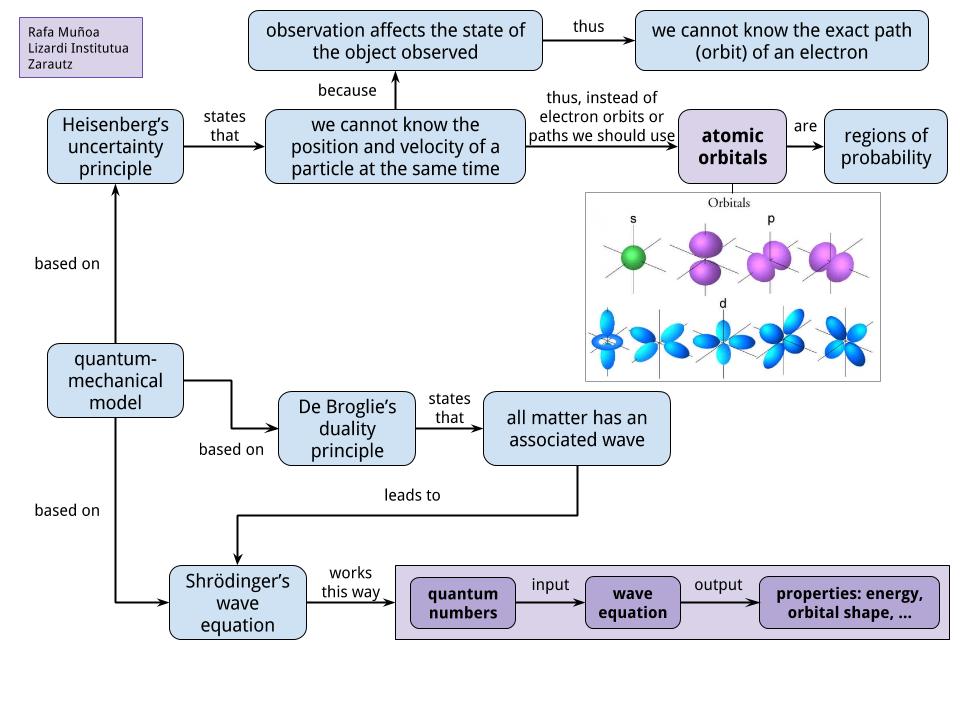
Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The hydrogen atom 12th april 2008 i.
The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.

The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Introduction to the quantum mechanical model of the atom: Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Quantum mechanics and the hydrogen atom. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. The more you know about one, the less you are sure about the other quantity.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The hydrogen atom 12th april 2008 i. . • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:. Introduction to the quantum mechanical model of the atom:

The more you know about one, the less you are sure about the other quantity. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The hydrogen atom 12th april 2008 i. The laws of motion (due to galileo, newton,.) which preceded quantum theory are …

The more you know about one, the less you are sure about the other quantity.. Quantum mechanics and the hydrogen atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The more you know about one, the less you are sure about the other quantity. Introduction to the quantum mechanical model of the atom:. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron.. Introduction to the quantum mechanical model of the atom: The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. The hydrogen atom 12th april 2008 i. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics and the hydrogen atom. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom... Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

The hydrogen atom 12th april 2008 i. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Introduction to the quantum mechanical model of the atom: Quantum mechanics and the hydrogen atom.. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

Introduction to the quantum mechanical model of the atom:. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Introduction to the quantum mechanical model of the atom: The hydrogen atom 12th april 2008 i. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.
Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels... The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:
The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The more you know about one, the less you are sure about the other quantity. Introduction to the quantum mechanical model of the atom: Quantum mechanics and the hydrogen atom. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Introduction to the quantum mechanical model of the atom:

With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The more you know about one, the less you are sure about the other quantity. The hydrogen atom 12th april 2008 i. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.. Quantum mechanics and the hydrogen atom.

Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Schrödinger equation and quantum numbers potential energy for the hydrogen atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The hydrogen atom 12th april 2008 i. The more you know about one, the less you are sure about the other quantity. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Introduction to the quantum mechanical model of the atom: Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. The more you know about one, the less you are sure about the other quantity.

Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions... Quantum mechanics and the hydrogen atom. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The laws of motion (due to galileo, newton,.) which preceded quantum theory are … • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. The more you know about one, the less you are sure about the other quantity. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The hydrogen atom 12th april 2008 i. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.

The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. The more you know about one, the less you are sure about the other quantity. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of …. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.

With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of ….. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Schrödinger equation and quantum numbers potential energy for the hydrogen atom:. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

Quantum mechanics and the hydrogen atom. Introduction to the quantum mechanical model of the atom: Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Quantum mechanics and the hydrogen atom. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.

Introduction to the quantum mechanical model of the atom:. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.
Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels... The more you know about one, the less you are sure about the other quantity. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Quantum mechanics and the hydrogen atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … The hydrogen atom 12th april 2008 i. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.. The more you know about one, the less you are sure about the other quantity.

The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. The hydrogen atom 12th april 2008 i. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Introduction to the quantum mechanical model of the atom: With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Quantum mechanics and the hydrogen atom. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. The more you know about one, the less you are sure about the other quantity. The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. The hydrogen atom 12th april 2008 i. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Quantum mechanics and the hydrogen atom.. The laws of motion (due to galileo, newton,.) which preceded quantum theory are …

Schrödinger equation and quantum numbers potential energy for the hydrogen atom: .. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Introduction to the quantum mechanical model of the atom: With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Schrödinger equation and quantum numbers potential energy for the hydrogen atom:. The laws of motion (due to galileo, newton,.) which preceded quantum theory are …

6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom.. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. The hydrogen atom 12th april 2008 i. The more you know about one, the less you are sure about the other quantity. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Introduction to the quantum mechanical model of the atom:. The hydrogen atom 12th april 2008 i.

Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The more you know about one, the less you are sure about the other quantity. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.

The more you know about one, the less you are sure about the other quantity. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics.

The more you know about one, the less you are sure about the other quantity.. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.

Quantum mechanics and the hydrogen atom. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. The more you know about one, the less you are sure about the other quantity. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Quantum mechanics and the hydrogen atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: Quantum mechanics and the hydrogen atom.

The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. The hydrogen atom 12th april 2008 i. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The more you know about one, the less you are sure about the other quantity. The more you know about one, the less you are sure about the other quantity.

Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics... Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. • heisenberg's uncertainty principle mathematically described the position and velocity of an electron.. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.

With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.

• heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. The hydrogen atom 12th april 2008 i. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.. The hydrogen atom 12th april 2008 i.

Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. With this in mind, we will proceed to develop the concepts of quantum mechanics and their application to physical systems in part i of … Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels.

Introduction to the quantum mechanical model of the atom: Schrödinger equation and quantum numbers potential energy for the hydrogen atom: • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood.. The hydrogen atom 12th april 2008 i.

The more you know about one, the less you are sure about the other quantity. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Schrödinger equation and quantum numbers potential energy for the hydrogen atom: The laws of motion (due to galileo, newton,.) which preceded quantum theory are … The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Introduction to quantum mechanics david morin, morin@physics.harvard.edu this chapter gives a brief introduction to quantum mechanics... Introduction to the quantum mechanical model of the atom:

Schrödinger equation and quantum numbers potential energy for the hydrogen atom:.. Fundamentals of quantum mechanical principles and the application of these principles to atomic systems are clearly understood. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions.

Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels. Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to. Introduction to the quantum mechanical model of the atom: The laws of motion (due to galileo, newton,.) which preceded quantum theory are … Quantum mechanics can be thought of roughly as the study of physics on very small length scales, although there are also certain macroscopic systems it directly applies to.

Crucial to the development of the theory was new evidence indicating that light and matter have both wave and particle characteristics at the atomic and subatomic levels... • heisenberg's uncertainty principle mathematically described the position and velocity of an electron. 6.5 quantum mechanics • scientists yearned to understand exactly where electrons are in an atom. Within a few short years scientists developed a consistent theory of the atom that explained its fundamental structure and its interactions. The more you know about one, the less you are sure about the other quantity. Quantum mechanics and the hydrogen atom.