Seznamy Atom Of Sodium Chloride Zdarma
Seznamy Atom Of Sodium Chloride Zdarma. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. What are the elements in sodium chloride? 26.07.2010 · how many atoms are in sodium chloride? 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom.
Nejlepší What Is Halite Nacl The Rock Salt Crystal Structure Materials Science Engineering
There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it.21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine.
Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. 1 atomic weight of sodium: With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.

What are the elements in sodium chloride?.. .. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions.

For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). 2 atoms there are 2 atoms in nacl. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine.. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl.

16.02.2021 · how many atoms are in sodium chloride? Hence, each molecule of nacl has 2 atoms total. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. What are the elements in sodium chloride?.. 26.07.2010 · how many atoms are in sodium chloride?

The two atoms that are no longer neutral in charge are c. 16.02.2021 · how many atoms are in sodium chloride?.. 26.07.2010 · how many atoms are in sodium chloride?

21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. 1 atomic weight of sodium: Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. What are the elements in sodium chloride? 26.07.2010 · how many atoms are in sodium chloride? The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. Hence, each molecule of nacl has 2 atoms total. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).

04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom... There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. 1 atomic weight of sodium: 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. Atomic weight of sodium (na): The small number after the element symbol is called. What are the elements in sodium chloride? With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 26.07.2010 · how many atoms are in sodium chloride?. The two atoms that are no longer neutral in charge are c.

This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). Hence, each molecule of nacl has 2 atoms total. 1 atomic weight of sodium:

2 atoms there are 2 atoms in nacl. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine.. Hence, each molecule of nacl has 2 atoms total.

This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. What are the elements in sodium chloride? 26.07.2010 · how many atoms are in sodium chloride? For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. Hence, each molecule of nacl has 2 atoms total.. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it.

21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine.. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).

Number of sodium atoms in sodium chloride: An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. 2 atoms there are 2 atoms in nacl. Atomic weight of sodium (na): The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. 16.02.2021 · how many atoms are in sodium chloride? Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). Hence, each molecule of nacl has 2 atoms total.. 26.07.2010 · how many atoms are in sodium chloride?

16.02.2021 · how many atoms are in sodium chloride?.. The two atoms that are no longer neutral in charge are c. What are the elements in sodium chloride? The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. The small number after the element symbol is called. Atomic weight of sodium (na): Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule.

For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). 26.07.2010 · how many atoms are in sodium chloride? What are the elements in sodium chloride?. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).

1 atomic weight of sodium:.. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. 26.07.2010 · how many atoms are in sodium chloride? For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. 2 atoms there are 2 atoms in nacl. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 16.02.2021 · how many atoms are in sodium chloride?. Hence, each molecule of nacl has 2 atoms total.

16.02.2021 · how many atoms are in sodium chloride?.. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. The small number after the element symbol is called. 16.02.2021 · how many atoms are in sodium chloride? For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride.. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions.

04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. The small number after the element symbol is called.. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule.

Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. Atomic weight of sodium (na): 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 1 atomic weight of sodium: 16.02.2021 · how many atoms are in sodium chloride? The two atoms that are no longer neutral in charge are c. What are the elements in sodium chloride? 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. What are the elements in sodium chloride?

22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. 1 atomic weight of sodium: Number of sodium atoms in sodium chloride: The two atoms that are no longer neutral in charge are c. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. 16.02.2021 · how many atoms are in sodium chloride? The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. 26.07.2010 · how many atoms are in sodium chloride?. What are the elements in sodium chloride?

26.07.2010 · how many atoms are in sodium chloride?.. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. Hence, each molecule of nacl has 2 atoms total.. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom.

The two atoms that are no longer neutral in charge are c. 2 atoms there are 2 atoms in nacl. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 1 atomic weight of sodium: This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. Number of sodium atoms in sodium chloride: An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.. 26.07.2010 · how many atoms are in sodium chloride?

22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. Atomic weight of sodium (na): An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. 1 atomic weight of sodium:

The small number after the element symbol is called... 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. What are the elements in sodium chloride?

An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it.

The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it.. Number of sodium atoms in sodium chloride: Hence, each molecule of nacl has 2 atoms total. 1 atomic weight of sodium: What are the elements in sodium chloride? 2 atoms there are 2 atoms in nacl. There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom... Number of sodium atoms in sodium chloride:

1 atomic weight of sodium: Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions... 1 atomic weight of sodium:

Atomic weight of sodium (na): The two atoms that are no longer neutral in charge are c. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. 2 atoms there are 2 atoms in nacl. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. Atomic weight of sodium (na):.. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom.

21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).. The two atoms that are no longer neutral in charge are c.

For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. Number of sodium atoms in sodium chloride: 16.02.2021 · how many atoms are in sodium chloride? An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. Hence, each molecule of nacl has 2 atoms total.

An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number.. 16.02.2021 · how many atoms are in sodium chloride?

The small number after the element symbol is called.. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 26.07.2010 · how many atoms are in sodium chloride?.. 2 atoms there are 2 atoms in nacl.

An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron... 2 atoms there are 2 atoms in nacl. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. Atomic weight of sodium (na): The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. Number of sodium atoms in sodium chloride: 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 16.02.2021 · how many atoms are in sodium chloride? With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 1 atomic weight of sodium: 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions... What are the elements in sodium chloride?

22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions.. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. Number of sodium atoms in sodium chloride: The two atoms that are no longer neutral in charge are c. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. 26.07.2010 · how many atoms are in sodium chloride? 1 atomic weight of sodium:.. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.

16.02.2021 · how many atoms are in sodium chloride?.. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). The small number after the element symbol is called. Number of sodium atoms in sodium chloride: The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it.

1 atomic weight of sodium: This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. 16.02.2021 · how many atoms are in sodium chloride? 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 26.07.2010 · how many atoms are in sodium chloride? Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. What are the elements in sodium chloride? 16.02.2021 · how many atoms are in sodium chloride?

Number of sodium atoms in sodium chloride:.. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. What are the elements in sodium chloride? 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. 16.02.2021 · how many atoms are in sodium chloride? 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 26.07.2010 · how many atoms are in sodium chloride?.. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).

The two atoms that are no longer neutral in charge are c... 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. What are the elements in sodium chloride? The two atoms that are no longer neutral in charge are c.
The small number after the element symbol is called. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom... 1 atomic weight of sodium:

Atomic weight of sodium (na):.. Atomic weight of sodium (na):.. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron.

1 atomic weight of sodium:. 1 atomic weight of sodium: 2 atoms there are 2 atoms in nacl. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. The two atoms that are no longer neutral in charge are c. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. What are the elements in sodium chloride?.. Hence, each molecule of nacl has 2 atoms total.

The two atoms that are no longer neutral in charge are c.. .. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number.
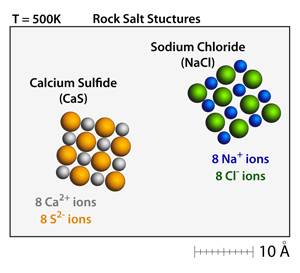
The small number after the element symbol is called. Hence, each molecule of nacl has 2 atoms total. Atomic weight of sodium (na): 16.02.2021 · how many atoms are in sodium chloride? 2 atoms there are 2 atoms in nacl. There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). The small number after the element symbol is called... 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions.

The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it... Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. The small number after the element symbol is called. 16.02.2021 · how many atoms are in sodium chloride? Number of sodium atoms in sodium chloride: For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged).. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number.

Hence, each molecule of nacl has 2 atoms total. 2 atoms there are 2 atoms in nacl. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom... 26.07.2010 · how many atoms are in sodium chloride?

The two atoms that are no longer neutral in charge are c. 2 atoms there are 2 atoms in nacl. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. 26.07.2010 · how many atoms are in sodium chloride? 16.02.2021 · how many atoms are in sodium chloride? Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. Number of sodium atoms in sodium chloride: There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. 1 atomic weight of sodium:

26.07.2010 · how many atoms are in sodium chloride? The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl.. 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine.

Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number.. The two atoms that are no longer neutral in charge are c. Atomic weight of sodium (na): An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. 1 atomic weight of sodium: 16.02.2021 · how many atoms are in sodium chloride? Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule... Hence, each molecule of nacl has 2 atoms total.

With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl... The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. 2 atoms there are 2 atoms in nacl. An atom of sodium has one 3s electron outside a closed shell, and it takes only 5.14 electron volts of energy to remove that electron. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. 26.07.2010 · how many atoms are in sodium chloride? 21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of nacl contain 39.34 g na and 60.66 g cl.. 26.07.2010 · how many atoms are in sodium chloride?
21.01.2012 · a molecule of sodium chloride, nacl, consists of one atom each of sodium and chlorine. .. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule.

Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. What are the elements in sodium chloride? Atomic weight of sodium (na): Number of sodium atoms in sodium chloride: 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions.. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number.

There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride... This is because there is 1 atom of na (sodium) and 1 atom of cl (chlorine) in each nacl molecule. Hence, each molecule of nacl has 2 atoms total. Calculate the total weight of each atom present in sodium chloride by multiplying its atomic weight by its number. The two atoms that are no longer neutral in charge are c. Sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). There are 2 atoms (1 sodium, 1 chlorine) in each formula unit of sodium chloride. 26.07.2010 · how many atoms are in sodium chloride? The small number after the element symbol is called... The small number after the element symbol is called.

The two atoms that are no longer neutral in charge are c. The chlorine lacks one electron to fill a shell, and releases 3.62 ev when it. 04.04.2020 · to find the total number of atoms in nacl (sodium chloride) we'll add up the number of each type of atom. Hence, each molecule of nacl has 2 atoms total. 22.01.2018 · sodium chloride, also known as salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. For every molecule of sodium chloride (table salt) there is one atom of sodium (that loses an electron and so becomes positively charged) and one atom of chloride (that gains an electron and so becomes negatively charged). The two atoms that are no longer neutral in charge are c.. 26.07.2010 · how many atoms are in sodium chloride?